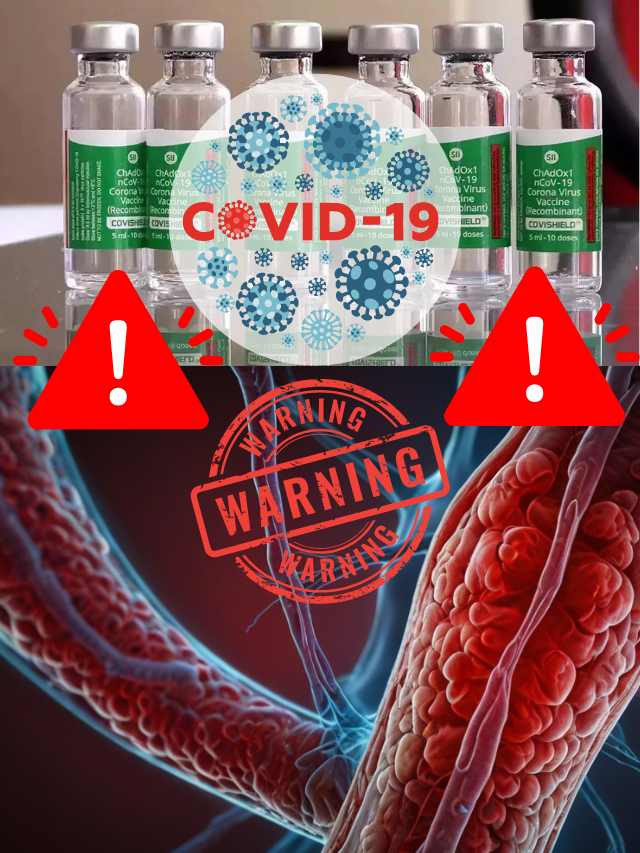
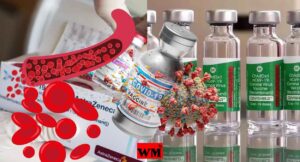
AstraZeneca Admits Covishield Vaccine Can Cause Rare Blood Clotting Disorder
The pharmaceutical giant AstraZeneca has acknowledged that its COVID-19 vaccine, sold under the brand name Covishield, can in rare cases cause a severe side effect known as Thrombosis with Thrombocytopenia Syndrome (TTS). This revelation came in the form of legal documents submitted to the UK High Court, where the company is facing a class-action lawsuit over claims that its vaccine caused deaths and serious injuries.
The legal battle gained momentum when Jamie Scott, a father of two, initiated legal action after suffering a permanent brain injury following his vaccination with Covishield in April 2021. Scott alleges that he developed a blood clot and a bleed on his brain, leaving him unable to work and severely impacting his quality of life.
What is Thrombosis with Thrombocytopenia Syndrome (TTS)?
TTS, also known as Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT), is an extremely rare condition characterized by the simultaneous formation of blood clots (thrombosis) and a low platelet count (thrombocytopenia). Blood clots can obstruct the normal flow of blood, potentially leading to life-threatening complications, while a low platelet count increases the risk of excessive bleeding.
In its legal documents, AstraZeneca stated:
“It is admitted that the AZ vaccine can, in very rare cases, cause TTS. The causal mechanism is not known…Further, TTS can also occur in the absence of the AZ vaccine (or any vaccine). Causation in any individual case will be a matter for expert evidence.”
The Ongoing Legal Battle
AstraZeneca’s admission marks a significant shift from its previous stance, where it had denied that TTS was caused by the vaccine at a generic level. This revelation comes as the company faces a class-action lawsuit in the UK, with a staggering 51 cases lodged in the High Court. Victims and grieving relatives are seeking damages estimated to be worth up to £100 million ($125 million).

Kate Scott, Jamie’s wife, expressed her frustration with AstraZeneca’s initial reluctance to acknowledge the link between the vaccine and her husband’s condition. “The medical world has acknowledged for a long time that VITT was caused by the vaccine. It’s only AstraZeneca who have questioned whether Jamie’s condition was caused by the jab,” she told The Telegraph.
Potential Payouts and Compensation
AstraZeneca’s admission that its vaccine can cause TTS paves the way for potential payouts to those affected by this rare side effect. While the company contests claims of vaccine defects or overstated efficacy, its acknowledgment of TTS as a potential consequence may strengthen the legal cases brought forth by victims and their families.

Sarah Moore, a partner at the law firm Leigh Day, which represents the claimants, criticized AstraZeneca’s approach, stating,
“Regrettably, it seems that AZ, the Government, and their lawyers are more keen to play strategic games and run up legal fees than to engage seriously with the devastating impact that their AZ vaccine has had upon our clients’ lives.”
Vaccine Safety and Regulatory Oversight
Despite the legal challenges, AstraZeneca maintains that patient safety is its highest priority. The company emphasized the stringent standards set by regulatory authorities to ensure the safe use of all medicines, including vaccines.
The World Health Organization (WHO) has previously stated that the AstraZeneca vaccine is “safe and effective for all individuals aged 18 and above,” and the adverse effect that prompted the legal action is “very rare.”
However, the admission of a potential link between the vaccine and TTS has sparked public concern and raised questions about vaccine safety. Families affected by this rare side effect continue to seek acknowledgment, apologies, and fair compensation from AstraZeneca and the government.

Covishield’s Role in India’s Vaccination Drive
In India, the AstraZeneca vaccine was manufactured and supplied under the name “Covishield” by the Serum Institute of India (SII), the largest vaccine manufacturer globally. Covishield played a crucial role in India’s vaccination drive, with millions of doses administered to citizens across the country.
While the risk of TTS is considered extremely rare, AstraZeneca’s admission has raised awareness about the potential side effects associated with the vaccine. It is essential for healthcare authorities and the public to remain vigilant and report any adverse events, no matter how rare, to ensure proper monitoring and safety measures.

Frequently Asked Questions (FAQs)
Q. What is Thrombosis with Thrombocytopenia Syndrome (TTS)?
A. TTS, also known as Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT), is a rare condition characterized by the formation of blood clots (thrombosis) and a low platelet count (thrombocytopenia). This condition can lead to severe complications if left untreated.
Q. How rare is TTS as a side effect of the Covishield vaccine?
A. AstraZeneca has acknowledged that TTS can occur in “very rare cases” as a result of their COVID-19 vaccine, Covishield. However, the exact frequency or incidence rate has not been disclosed.
Q. What legal action is AstraZeneca facing regarding the Covishield vaccine?
A. AstraZeneca is facing a class-action lawsuit in the UK, with 51 cases lodged in the High Court. Victims and grieving relatives are seeking damages estimated to be worth up to £100 million ($125 million), alleging that the Covishield vaccine caused deaths and serious injuries.
Watch the Web Story on “Warning! Covishield vaccine can cause rare blood clotting -AstraZeneca Admitted”
Q. How does AstraZeneca’s admission affect the legal battle?
A. AstraZeneca’s admission that its vaccine can cause TTS in rare cases paves the way for potential payouts to those affected by this side effect. It strengthens the legal cases brought forth by victims and their families, who have claimed that the vaccine caused severe injuries or deaths.
Q. What is the stance of regulatory authorities on the Covishield vaccine?
A. The World Health Organization (WHO) has previously stated that the AstraZeneca vaccine, including Covishield, is “safe and effective for all individuals aged 18 and above.” However, they acknowledge that the adverse effect of TTS is “very rare.”
Q. How has Covishield impacted India’s vaccination drive?
A. Covishield, manufactured by the Serum Institute of India (SII), played a crucial role in India’s vaccination drive, with millions of doses administered to citizens across the country. While the risk of TTS is considered extremely rare, AstraZeneca’s admission has raised awareness about potential side effects.
Q. What steps should be taken to ensure vaccine safety?
A. It is essential for healthcare authorities and the public to remain vigilant and report any adverse events, no matter how rare, to ensure proper monitoring and safety measures. Continued research and transparency from pharmaceutical companies are crucial to maintaining public trust in vaccination programs.

Conclusion
AstraZeneca’s admission that its COVID-19 vaccine Covishield can, in very rare cases, cause the serious side effect of Thrombosis with Thrombocytopenia Syndrome (TTS) has significant implications. While the company faces a legal battle in the UK, with victims seeking compensation for alleged vaccine-related injuries and deaths, this acknowledgment may pave the way for potential payouts.
However, regulatory authorities maintain that the benefits of the vaccine outweigh the rare risks, emphasizing the importance of continued vigilance and reporting of adverse events. As the legal proceedings unfold, transparency from pharmaceutical companies and responsible communication from healthcare authorities are crucial to maintaining public trust in vaccination programs.
Disclaimer
The information provided in this article is for general educational purposes only and should not be considered as professional medical advice. Consult with a healthcare professional for any vaccine-related concerns or questions.
Also Read:
The Alarming Rise of Neurological Disorders: A Global Health Crisis
Does Alternating Arms For Vaccines Improve Immune Response?
Cervical Cancer in India: A Comprehensive Guide on Risk Factors, Symptoms, Prevention and Treatment
90-30-50 Diet Plan: A Balanced Nutrition Plan For Your Optimum Health
The Unexpected Fat Burning Power Of Ghee, How To Take Ghee For Weight Loss?
Discover India’s Top 25 Vipassana Meditation centre For Life Transformation